Medical Applications & Breakthrough Research
Introduction: Rigorous Verification Builds Confidence – A Solid Bridge from Laboratory to Clinic
Animal experiments are a key link in evaluating the safety and effectiveness of new medical technologies, serving as an indispensable bridge between basic research and clinical applications. We chose the New Zealand white rabbit as our primary soft tissue regeneration research model due to its physiological, anatomical, and biomaterial response similarities to humans, as well as ease of operation and management. All animal experiments strictly comply with internationally recognized animal welfare and experimental operation guidelines (e.g., AAALAC certification standards or equivalent) and have received approval from the Institutional Animal Care and Use Committee (IACUC).
Validated Applications: Animal Experiment Successes
Application 1: Absorbable Surgical Sutures – Accelerating Wound Healing & Optimizing Repair Quality
Background Challenge: Traditional surgical sutures, especially non-absorbable ones, can cause persistent foreign body reactions, inflammation, and bacterial colonization sites, affecting wound healing quality and leading to scar proliferation or delayed healing. Absorbable surgical suture such as catgut, synthetic polymer sutures, elicit an immune or inflammatory response. Ideal absorbable sutures should offer good biocompatibility, sufficient initial tensile strength, predictable absorption time, and actively promote tissue repair.
Experimental Design: A full-thickness incision model on the rabbit's back skin was established. Animals were randomized into two groups: the experimental group used absorbable sutures made of our soluble collagen material; the control group used commercially available PGA sutures. Wound healing was observed at 3, 7, and 18 days post-surgery, assessing general appearance, histology (inflammatory cell infiltration, collagen deposition, epidermal regeneration), and wound tensile strength.
Core Findings & Data: Compared to the control group, the collagen suture group showed faster wound healing and significantly reduced early inflammation. Histological examination revealed earlier neovascularization, more orderly collagen fiber deposition, more complete epidermal regeneration, and lower scar formation. Specific data is detailed in our medRxiv preprint: https://doi.org/10.1101/2025.05.19.25325989
Conclusion & Significance: This soluble collagen-based surgical suture significantly accelerates wound healing, reduces inflammation, and promotes high-quality skin regeneration (potentially reducing scar formation). Its excellent biocompatibility and controllable degradation make it highly promising for various surgical operations, especially in fields requiring high healing quality like plastic and microsurgery.
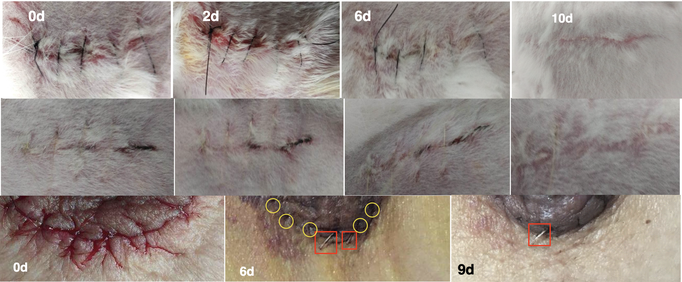
Figure 1 1st row: Black suture applied to the rabbit's back skin wound. 2nd row: Transparent suture applied to the rabbit's back skin wound. 3rd row: Transparent suture applied to the patient's chest skin wound. Both show good wound healing effects.
Application 2: Regenerative Artificial Tendon – World's First! Successfully Overcoming Large Tendon Defect Repair
Background Challenge: Large-area tendon defects, common after severe injuries or tumor resection, are a major challenge in orthopedics and sports medicine. Existing treatments (autografts, allografts, synthetic materials) have significant limitations, often leading to poor functional recovery.
Experimental Design: A large-segment (2cm) defect model of the rabbit Achilles tendon was created. Then received a tissue-engineered artificial tendon constructed from our biomimetic material. Evaluations were performed at 4, 8, 12, and 20 weeks post-operation (gross observation, histology, mechanical strength etc.).
Core Findings & Data: We achieved the world's first successful functional regeneration of large-area tendon defects in rabbits. The defect area was gradually filled with new tissue resembling normal tendon. Histology showed oriented Type I collagen fibers, tendon-like cells, and good vascularization. Biomechanical properties significantly improved, approaching normal tendon levels by 20 weeks. Details are in our preprint: bioRxiv:
Conclusion & Significance: This is a historic, world-class breakthrough in tendon regeneration. It demonstrates that large-area tendon defects can be effectively repaired and functionally regenerated through tissue engineering, offering a revolutionary treatment strategy for severe tendon injuries.

Figure 2 Images a, b, c: Artificial tendon transplantation; d, d1, d2: 20-week regenerated tendon with a white outer layer; d2: separated outer white layer; e, e1, e2: half of the 20-week tendon; e:regenerated tendon with nylon suture and collagen fiber; e1: cross-section; e2: regenerated tendon without nylon suture and collagen fiber; green arrow: regenerated tendon; white arrow: native autologous tendon.
Application 3: Functional Artificial Muscle – World's First! Effectively Addressing Volumetric Muscle Loss (VML)
Background Challenge: Volumetric Muscle Loss (VML) results in permanent absence of large skeletal muscle tissue, leading to severe dysfunction. Current clinical methods for VML repair are lacking.
Experimental Design: A standardized VML model (3x3cm abdominal wall muscle removal) in rabbits was established. The experimental group received a muscle scaffold of our soluble collagen material (optionally preloaded with growth factors or combined with stem cells). The control group received a PP (polypropylene) mesh. Evaluations occurred at 4, 8, 16, 24, and 32 weeks (regenerated muscle volume/weight, histology, muscle fiber density/maturity, capillary network, in-body muscle strength).
Core Findings & Data: This study also achieved a world's first major breakthrough: successful regeneration of large functional skeletal muscle in the rabbit VML model. The VML defect was filled with new muscle tissue. Histology confirmed new, orderly muscle fibers and a rich capillary network. Muscle strength recovery was significant compared to controls. See our preprint: bioRxiv:
Conclusion & Significance: Landmark progress in VML regeneration. It powerfully demonstrates that large, vascularized, and functional skeletal muscle tissue can be regenerated, offering unprecedented hope for VML patients.
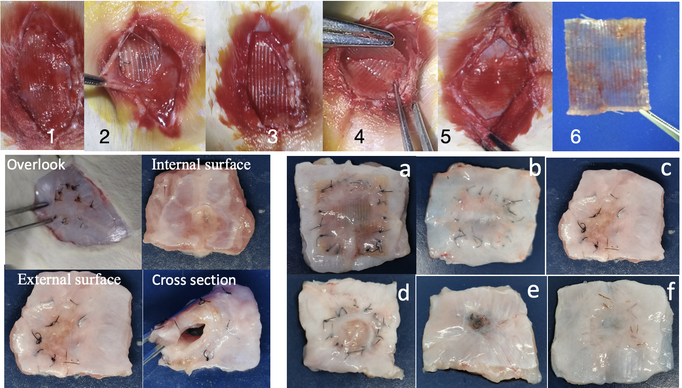
Figure 3: Hernia Patch Transplantation and Abdominal Wall Regeneration. Upper Panel: In vivo hernia patch 17 days after transplantation. Lower Left Panel: Abdominal wall regeneration 16 weeks after transplantation. Bottom Right Panel: Regenerated abdominal wall in chronological order (nylon sutures 4, 8, 16, 24, 32 weeks); f: collagen suture - 32 weeks)
Application 4: Guiding Artificial Nerve – Promoting Nerve Repair and Restoring Tissue Function
Background Challenge: Repair of peripheral nerve long-segment defects (>1-2cm) is a classic neurosurgical problem. Autografts have limitations, and existing Nerve Guidance Conduits (NGCs) need improvement.
Experimental Design: A 20mm long-segment defect model of the rabbit sciatic nerve was established. Then it was repaired by using an artificial neural conduit made of our soluble collagen material. Evaluations were performed at 4, 8, 12, 24, and 36 weeks.
Core Findings & Data: The material effectively guided and supported long-distance axon regeneration. Histology showed regenerated nerve tracts. Functional recovery was good. Details are in our preprint: bioRxiv:
Conclusion & Significance: This soluble collagen-based artificial nerve catheter is a promising new biomaterial for long peripheral nerve defects. It effectively guides axon regeneration, promotes myelination, and restores nerve function, offering advantages over autografts and some existing NGCs.

Figure 4 Regenerated nerves in chronological order (8, 12, 24, 36 weeks) and rabbit autologous sciatic nerve
Summary of Research Results & Scientific Value:
- Common Advantages: Excellent biocompatibility, strong tissue regeneration induction, remarkable repair effects (structural and functional).
- Scientific Significance: Validates material superiority, provides new insights into soft tissue regeneration mechanisms, and challenges traditional limitations (e.g., that large muscles are difficult to regenerate functionally via tissue engineering).
- Clinical Transformation Potential: Solid animal data provides a strong foundation for preclinical research and clinical trial design for human applications.
Unified Link to Preprints: For more detailed experimental data and methodological descriptions, please refer to our preprints on mediRxiv, and bioRxiv:
- Tendon Regeneration Research
- VML Regeneration Research
- Nerve Regeneration Research
- Surgical Sutures Research
Reshaping Medical Fields: Future Application Expansion
Our soluble collagen-based biomimetic regenerative materials represent a platform technology with wide applicability. We are committed to deepening verified applications and expanding into new high-need areas.
Deepening & Market Expansion of Verified Applications:
- High-end Sutures: For plastic surgery, microsurgery, cardiovascular surgery, neurosurgery.
- Broad Tendon/Ligament Repair: Acute trauma (rotator cuff, Achilles, ACL), chronic strain, degenerative lesions.
- Diverse VML Scenarios: Trauma, tumor resection, congenital muscle dysplasia, muscular dystrophy (as carrier), muscle atrophy.
- Refined Nerve Repair Applications: NGCs for various nerve diameters, types, and lengths; nerve transplantation, neuroma prevention.
Prospective New Application Fields:
1. Artificial Small-Caliber Blood Vessels (<6mm):
- Clinical Pain Points: High failure rates of current synthetic Small-Diameter Grafts (SDGs) due to thrombosis and intimal hyperplasia. Autograft limitations.
- Material Potential: Soluble collagen's inherent low thrombogenicity and promotion of endothelialization. Construction of biomimetic multi-layer tubular scaffolds to mimic natural vessel structure and mechanics, aiming for rapid endothelialization and improved patency.
- Expected Benefits: Superior alternative to existing SDGs and autografts, reducing surgical risk and complications for millions with cardiovascular diseases.
- Market Size Indication: The global cardiovascular market is vast, with millions of bypass surgeries annually. The small-caliber artificial blood vessel market is projected for >10% CAGR.
2. Tissue-Engineered Trachea/Bronchi:
- Clinical Pain Points: Long-segment airway stenosis, post-tumor defects, congenital malformations. Limitations of current repair methods.
- Material Potential: Tubular collagen scaffold with radial support and biocompatibility, designed to guide epithelial and cartilage cell regeneration, forming functional tissue-engineered airways.
- Expected Benefits: Potential radical treatment, avoiding tracheostomy, improving respiratory function and quality of life for patients with complex airway diseases.
- Market Size Indication: A niche but critical unmet medical need with significant social value.
3. Regenerative Artificial Esophagus:
- Clinical Pain Points: Challenges in esophageal reconstruction post-esophagectomy (e.g., for cancer) using gastric/colonic conduits; issues with chemical burns or congenital defects.
- Material Potential: Tubular collagen scaffold with biomimetic multi-layer structure, loaded with growth factors to guide in-situ regeneration of esophageal tissue with normal peristalsis and barrier function.
- Expected Benefits: Less traumatic, fewer complications, higher quality of life, and closer to physiological function for patients with esophageal defects.
- Market Size Indication: High incidence of esophageal cancer; persistent demand for improved reconstruction techniques.
4. Advanced Cell Culture Substrate (Replacing Temperature-Sensitive Polymers like PIPAAm/PNIPAAm):
- Clinical Pain Points of PIPAAm: Synthetic polymers like PIPAAm/PNIPAAm, while avoiding enzymatic damage for cell sheet harvesting, differ significantly from the natural ECM in chemical composition, 3D structure, and physical properties. This can negatively impact stem cell self-renewal and differentiation potential, as they lack complex biological signals (ligands, growth factor binding sites, appropriate rigidity) provided by natural ECM. This can lead to unexpected deviations in stem cell differentiation and difficulty in maintaining a "pluripotent" state long-term.
- Superiority of Soluble Collagen Sheets: Medical-grade soluble collagen sheets offer unparalleled biocompatibility and very low immunogenicity, promoting cell adhesion, spreading, migration, and proliferation. The 3D network structure provides an excellent microenvironment for cell growth. Its degradation products (small peptides and amino acids) are reusable by the body, avoiding foreign material residue. Critically, cell sheets cultured on collagen materials can be transplanted as a "complex" of cells and their biologically active matrix, avoiding the cooling and peeling steps that can damage cells and induce physiological stress. This preserves cell phenotype, protein expression, and specific biological functions, which is especially important for sensitive stem cells and overall therapeutic efficacy. Reports indicate collagen matrices support cell adhesion, proliferation, and differentiation better than glass or plastic substrates.
Other Potential Directions: Full-thickness skin substitute, dural/spinal patch, bladder tissue engineering, myocardial patch/heart valve tissue engineering, cartilage tissue engineering.
Product Blueprint: Implant Product Matrix
Our high-strength soluble collagen materials can be processed into various forms for a wide range of implantable medical devices:
By Material Form:
- Membrane/Sheet Forms: Hernia repair patches, skin repair membranes/artificial dermis, anti-adhesion barriers, dural/spinal patches, pericardial patches, oral/GTR/GBR membranes, pelvic floor reconstruction meshes.
- Line/Fiber Forms: Absorbable surgical sutures, core braiding material for artificial tendons/ligaments, reinforced suture lines, oriented fiber scaffolds for tissue engineering.
- Tubular Products: Artificial nerve conduits, artificial blood vessels, artificial ureters/urethras, artificial bile ducts/esophagi/tracheas.
- Sponge/Hydrogel/Granule Forms: Hemostatic sponges/powders, bone defect fillers, injectable bone repair materials, tissue engineering scaffolds, cell delivery/repair, drug release carriers.
- Woven/Complex 3D Constructs: Braided artificial tendons/ligaments, customized 3D printed/bio-woven tissue/organ scaffolds (e.g., for blood vessels, esophagus, trachea).
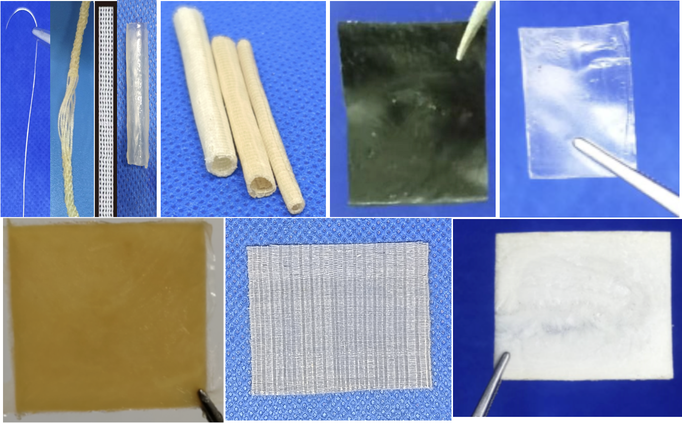
Figure 5: Biomimetic medical materials derived from soluble collagen exhibit a diverse range of characteristics, encompassing colors( brown, black, transparent, and white), shapes(pipes, nets, pieces, and threads), and textures (sponges, membranes, and pieces).
By Target Tissue/Organ Application:
- Nervous System: Nerve conduits, dural patches.
- Musculoskeletal System: Artificial tendons/ligaments, muscle repair sheets, bone/cartilage repair materials.
- Cardiovascular System: Pericardial patches, small-caliber blood vessels, stent coatings, valve materials.
- Skin & Soft Tissue: Skin substitutes, sutures, wound dressings, hernia repair, breast reconstruction.
- Digestive System: Intestinal/gastric wall reinforcement, esophageal/tracheal/bile duct scaffolds.
- Genitourinary System: Artificial urethra/ureter, bladder wall repair, pelvic floor reconstruction.
- Facial & Sensory Organs: Oral/GTR/GBR membranes, eardrum repair, ophthalmic implants, nasal/auricular cartilage scaffolds.
Nugget Blue Sea: Market Value Evaluation and Replacement Potential
The global medical implant market is substantial and growing. Custom Market Insights (CMI) reported the market reached $112.6 billion in 2023, projecting growth to $203.6 billion by 2032 (CAGR 6.8%). GlobeNewswire noted a 2023 market size of $110.25 billion, expected to reach $214.76 billion by 2033 (CAGR 6.90%). Our technology targets key segments within this market.
Analysis of Key Potential Implant Products:
Absorbable Surgical Sutures:
- Global Market Size (Annual): Absorbable segment approx. $4-6 Billion.
- Our Advantages: Extremely low immunogenicity/inflammation, excellent biocompatibility, promotes healing, reduces scarring.
- Market Substitution Potential: High, especially in premium markets (plastic surgery, pediatrics).
- Estimated Commercial Value Potential (Our Material): $200M - $900M annually (assuming 5-15% market share).
Hernia Repair Mesh/Patch:
- Global Market Size (Annual): Hernia repair devices approx. $5-7 Billion.
- Our Advantages: Excellent biocompatibility, low chronic inflammation, guides tissue regeneration, fully absorbable.
- Market Substitution Potential: High, particularly for patients seeking higher quality of life and in contaminated fields.
- Estimated Commercial Value Potential (Our Material): $500M - $1.4B annually (assuming 10-20% market share).
Artificial Nerve Conduit (Nerve Guidance Conduit):
- Global Market Size (Annual): NGC segment approx. $300-500 Million.
- Our Advantages: Excellent biocompatibility, promotes axon growth/myelination, controllable degradation.
- Market Substitution Potential: High, potential alternative to autografts for certain defects.
- Estimated Commercial Value Potential (Our Material): $45M - $150M annually (assuming 15-30% market share).
Notes on Market Value Evaluation: These are preliminary estimates subject to clinical trial outcomes, pricing, regulatory approvals, and competition.
Market Value Overview and Strategic Outlook:
Our high-strength soluble collagen material, with its unique combination of biological characteristics, is poised to be a platform technology for next-generation medical regenerative materials. Its core value lies in trauma repair, tissue reconstruction, and regenerative medicine.
Key Success Factors: Conclusive clinical evidence, stable large-scale production & QC, clear regulatory pathway, cost-effectiveness, effective marketing & academic promotion, strong IP.
Challenges: Lab-to-industrial scale-up, long-term in vivo safety/efficacy verification, competition, technical bottlenecks for complex organs.
Key Points: Medical Applications
- Successfully validated in animal models for sutures, tendons (world-first), muscles (VML, world-first), and nerves.
- Strong potential for expansion into artificial small-caliber blood vessels, trachea, esophagus, and as an advanced cell culture substrate.
- Extensive product blueprint covering various material forms and target tissues.
- Significant market value in key implant categories with high replacement potential.
